JUUL LABS INITIATES TARGETED CAMPAIGN IN SOUTH FLORIDA TO ADDRESS ILLICIT TRADE AND PROMOTE A MORE RESPONSIBLE, REGULATED MARKETPLACE FOR VAPOR PRODUCTS
Company News, Underage Use Prevention
February 18, 2021
Juul Labs recently initiated an enforcement campaign in South Florida to identify retailers trafficking counterfeit and other illicit vapor products, obtain intelligence on upstream suppliers, and take expansive enforcement action to address a growing black market. An emerging public health issue, illicit vapor products undermine underage-prevention measures and may present additional health and safety risks for adult consumers.
Juul Labs’ Brand Protection Team conducted an investigation of 917 retailers in Miami-Dade County and Broward County, representing a major urban area that comprises various classes of retailers, including convenience stores and specialty vape shops. These South Florida counties are also in close proximity to a U.S. port-of-entry and international-mailing facility — known entry points for the importation of illicit products. The team, with the support of a third-party compliance auditor, conducted product surveillance and obtained samples across retailers. The samples were then evaluated to determine whether they were illicit products. Ultimately, the product surveillance identified 30 retail outlets (or, about 3.3% of all surveilled outlets), as selling illicit counterfeit, diverted, or unauthorized JUUL-compatible products.
Counterfeit JUUL products are designed and marketed to mimic authentic JUUL products. Diverted JUUL products are initially manufactured under Juul Labs’ control and produced for lawful distribution in an intended market, but are illegally diverted into a different, non-compliant market without the company’s authorization. Unauthorized JUUL-compatible products are designed and marketed to be used with authentic JUUL products without the company’s authorization. Counterfeit and compatible products violate intellectual-property rights and may raise additional health and safety risks given their untested ingredients, lack of manufacturing and quality controls, and unsanitary conditions in which they are produced.
Illicit vapor products may actively undermine underage-prevention measures given their ease of access. As FDA has noted, “to the extent that such products are sold through nontraditional retail channels, such as social sources or online commercial marketplaces that do not include age-verification requirements, they pose an increased risk of being accessed by minors.” Moreover, FDA’s compliance-check inspections and Juul Labs’ internal Mystery Shops and market surveillance find that retailers carrying illicit products are less likely to comply with age-verification requirements.
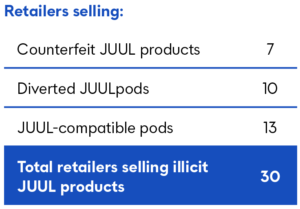
Of the 30 outlets in South Florida selling illicit products, 6 outlets sold counterfeit JUULpods, primarily offered in illegally marketed flavors, while 1 sold counterfeit JUUL devices. 10 outlets sold diverted JUULpods, primarily diverted from Canadian and Russian markets, and 13 outlets sold illegal and unauthorized compatible pods, with the majority of these compatible brands subject to International Trade Commission exclusion orders.
Insights into these illicit activities will inform broad enforcement actions against these violating retailers, including cease-and-desist letters, training and education, and litigation as needed.
But these actions are just a starting point: The Brand Protection Team will collect additional records and information from the violating retailers to identify upstream suppliers and sources of the illicit products. This will result in further enforcement action to disrupt the illicit trade of black-market vapor products that are impacting local communities.
Juul Labs will deliver these findings to law enforcement authorities and support their efforts to bring legal action. We will also continue to share these findings with relevant regulators and policymakers in our efforts to combat the illicit trade of vapor products through additional enforcement and effective public policy.
“We need to be a responsible and trusted steward of vapor products,” said Adrian Punderson, Vice President of Brand Protection at Juul Labs. “As such, it is our obligation to support enforcement against illicit and illegal products as we strive to reset the vapor category and earn a license to operate in society.”
The targeted South Florida campaign is the latest example of Juul Labs’ commitment to use its global enforcement program to aggressively disrupt the illicit trade of vapor products and work to create a more responsible marketplace for current adult users.
Other Posts
January 29, 2025
U.S. INTERNATIONAL TRADE COMMISSION PROTECTS JUUL LABS’ AMERICAN PATENTS
The U.S. International Trade Commission (ITC) issued a decision today affirming an Administrative Law Judge’s ruling that Altria’s NJOY ACE e-vapor products infringe four…
October 2, 2024
K.C. CROSTHWAITE DELIVERS KEYNOTE ADDRESS ON RESPONSIBLE INNOVATION IN E-VAPOR INDUSTRY
On September 26th, Juul Labs’ Chairman & Chief Executive Officer, K.C. Crosthwaite, delivered the keynote address at the 2024 Global Tobacco & Nicotine Forum,…
July 29, 2024
JUUL LABS STATEMENT ON SETTLEMENT WITH FORMER NOTEHOLDERS
Juul Labs announced today the settlement with a group of noteholders that confirms the conversion of the convertible notes to equity, and provides an…
