PEER-REVIEWED STUDY COMPARES NICOTINE DELIVERY OF COMBUSTIBLE CIGARETTES TO JUUL PRODUCTS AVAILABLE IN UNITED STATES, CANADA, AND EUROPE
Research & Analysis
January 25, 2021
A new study from Juul Labs published in the journal Nicotine & Tobacco Research found that the nicotine delivery of JUUL products available in the United States and Canada (59 mg/mL or 5.0% nicotine by weight) more closely resembles the nicotine delivery and experience of cigarette smoking than JUUL products available in the European Union, which contain 18 mg/mL and/or 9 mg/mL of nicotine. Researchers posited that heavier and more dependent smokers in particular may require the greater nicotine delivery of the higher nicotine concentration JUULpods (59 mg/mL) in order to successfully transition away from cigarettes.
Providing a satisfying nicotine effect and experience compared to combustible cigarettes is critical to facilitate an adult smoker’s transition from cigarettes to a potentially less harmful alternative product. Under the Tobacco Products Directive (TPD) regulatory framework in the European Union, the current maximum allowable concentration of nicotine in e-cigarettes is 20 mg/mL. Existing research shows that nicotine limits on e-cigarettes may limit the ability for such products to switch adult smokers off of combustible cigarettes in the EU.
The new study, which consisted of 24 adult smokers, assessed the nicotine delivery and subjective effects of combustible cigarettes compared to the JUUL System with three nicotine concentrations:
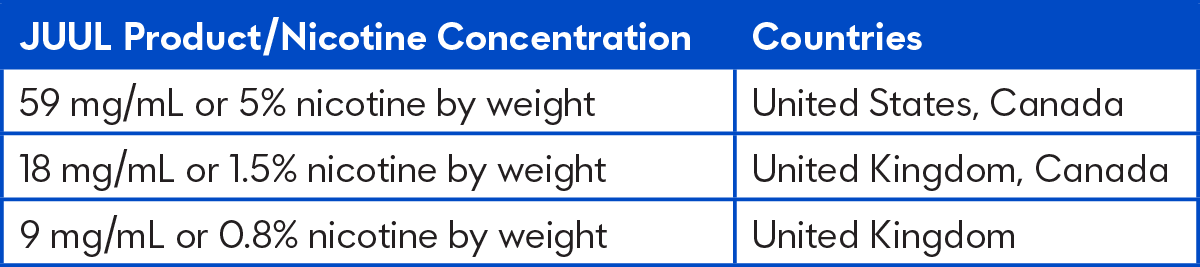
At each of five study visits, participants used one of four JUUL products or smoked their usual brand of cigarette during controlled (10 puffs) and ad libitum use (5 minutes) sessions. Blood samples were collected, and levels of nicotine in the bloodstream were measured for each study product. Subjective effects, including relief of craving for cigarettes and withdrawal symptoms, were assessed 30 minutes after participants used each product.
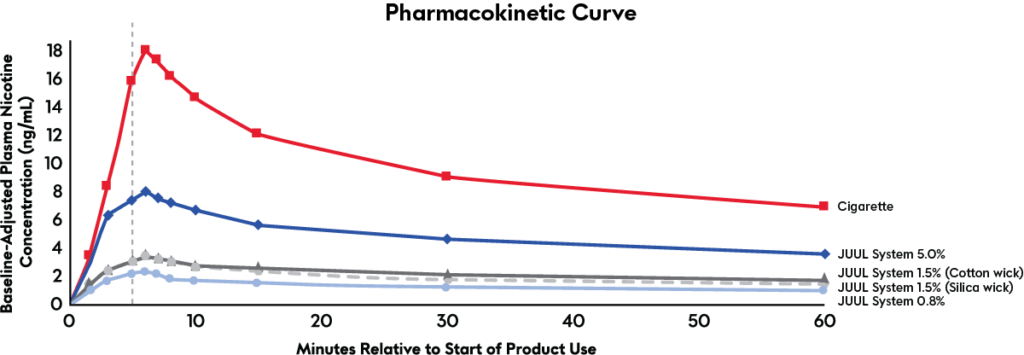
This pharmacokinetic curve illustrates the nicotine absorption profile in the bloodstream for the JUUL System with three concentrations of nicotine compared to a combustible cigarette.
The higher concentration (59 mg/mL or 5.0%) JUUL product delivered significantly greater levels of nicotine and significantly reduced craving and withdrawal compared to the JUUL with 18 mg/mL and 9 mg/mL nicotine concentrations. Researchers concluded that the lower nicotine delivery and craving relief from the 18 mg/mL and 9 mg/mL JUULpods available in the EU may limit the product’s ability to provide a satisfying alternative to cigarette smoking — particularly for more dependent adult smokers living in that region.
Notably, results from a Juul Labs behavioral study found that while rates of complete switching from cigarettes to JUUL products among adult smokers who purchase JUUL products in the United Kingdom are encouraging, they are lower than switch rates among similar smokers in the United States and Canada, where the higher concentration products are available. A longitudinal study presented last year found that after adjusting for key characteristics such as demographics and smoking history, rates of switching away from combustible cigarettes among adult smokers in the United States and Canada, where JUUL products are available in 59 mg/mL, were higher than in the United Kingdom, where the highest concentration available is 18 mg/mL.
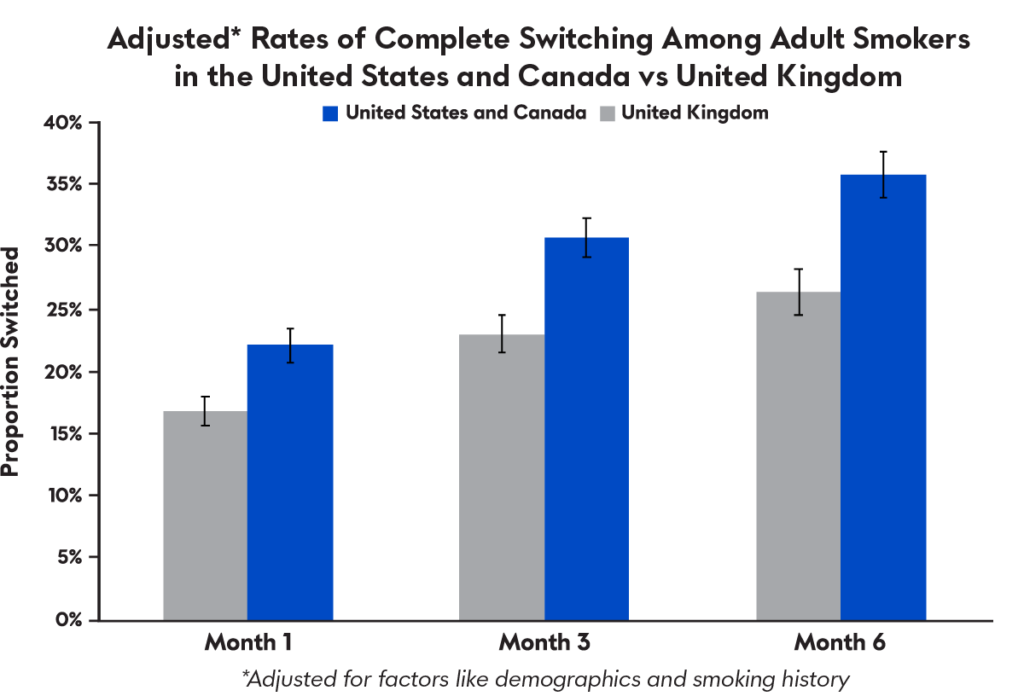
Taken together, these results suggest that the availability of JUUL products (and other e-cigarettes) in a variety of nicotine concentrations — some greater than 20 mg/mL — may be associated with increased switching among adult smokers. Heavier and more dependent smokers, in particular, may require the greater nicotine delivery of 59 mg/mL JUUL product to successfully transition away from combustible cigarettes.
“When considering laws and regulations governing nicotine concentration in ENDS, policymakers should bear in mind that the availability of a variety of alternative nicotine products may facilitate even more smokers transitioning away from cigarettes,” said Mark Rubinstein, Vice President of Global Scientific Affairs at Juul Labs.
The study, entitled “An Open-Label, Randomised, Controlled, Crossover Study to Assess Nicotine Pharmacokinetics and Subjective Effects of the JUUL System with Three Nicotine Concentrations Relative to Combustible Cigarettes in Adult Smokers” was published in the journal Nicotine & Tobacco Research.
Juul Labs is fully committed to the Premarket Tobacco Product Application (PMTA) process in the United States, and to that end has built a robust scientific research program to help assess the harm-reduction potential of JUUL products, including their impact on the individual user, their ability to move adult smokers away from combustible cigarettes, and the net-population impact on public health. We are committed to working cooperatively with regulators, public health officials, and other stakeholders to preserve the historic opportunity to transition adult smokers away from combustible cigarettes, while combating underage use.
Juul Labs will continue to share results from its science and research program transparently with the public health community as it works to support the scientific basis for the category, as well as future regulatory filings.
About Juul Labs
Juul Labs’ mission is to transition the world’s billion adult smokers away from combustible cigarettes, eliminate their use, and combat underage usage of our products. We believe that vapor products can offer adult smokers an alternative to combustible cigarettes and, in so doing, reduce the harm associated with tobacco. Nicotine is addictive and can potentially be harmful. It would be best if no one used any nicotine product. Anyone who smokes should quit. Adult smokers who have not successfully quit should completely switch to potentially less harmful alternative nicotine products. Juul Labs does not want any non-nicotine users, especially those underage, to try our products, as they exist only to transition adult smokers away from combustible cigarettes. JUUL products are not intended to be used for smoking cessation or other therapeutic purposes.
For the general public and policymakers looking for more information about the company, please visit www.juullabs.com.
For scientists, members of the public health community, regulators and policymakers looking to learn more about Juul Labs’ scientific research, please visit www.juullabsscience.com.
Other Posts
October 25, 2024
ROBYN GOUGELET DELIVERS REMARKS DURING A PUBLIC MEETING HOSTED BY FDA AND NIH
On October 21, Juul Labs’ Vice President of U.S. Regulatory Affairs, Robyn Gougelet, delivered remarks during a joint public meeting hosted by the U.S….
March 17, 2023
JUUL LABS PUBLISHES WHITE PAPER EXAMINING THE REAL-WORLD IMPACT OF ENDS PRODUCTS FOR ADULT SMOKERS
In a new white paper entitled “The Real-World Impact of ENDS for Adult Smokers: Tobacco Harm Reduction Through Real-World Data and Evidence,” Juul Labs has reviewed and compiled the latest science and evidence demonstrating the positive real-world impact of ENDS products for adult smokers.
March 17, 2022
PEER-REVIEWED STUDY FINDS BANNING VAPOR PRODUCTS MAY LEAD TO INCREASED CIGARETTE SALES
This research shows that policies that significantly restrict vapor products are likely deterring current adult smokers from switching and driving former adult smokers back to combustible cigarettes, which remain the leading cause of preventable death in the U.S. and worldwide.
